This 2013 study in the journal of Gastroenterology
No Effects of Gluten in Patients With Self-Reported Non-Celiac Gluten Sensitivity After Dietary Reduction of Fermentable, Poorly Absorbed, Short-Chain Carbohydrates
has been heralded as definitive proof that avoiding gluten does not confer any health benefits, save for non-Celiacs (maybe). This is a tenuous claim at best and most likely results from:
- a lack of attention to study design details
- discounting past in vitro studies & in vivo studies in mice and humans
- an annoyance towards those behaviors associated with ‘going gluten-free’
Before exploring why this study reframes the gluten question rather than dismisses it, delineating the specific hypothesis being tested is necessary. The authors ask:
“How do people respond to different whey and gluten challenges in the context of a diet lowered in FODMAPs?”
Uh, what are FODMAPs? They are fibers of the short chain Fermentable Oligo-Di-MonosAccharides & Polyols sort.
So who are we talking about? Entry criteria:
To find data pertaining to this question, they enrolled 37 non-Celiac subjects diagnosed with irritable bowel syndrome (IBS) and reporting stable improvements in their symptoms for at least 6 weeks when going on a gluten-free diet (GFD) prior to the study.
What did they feed to who and when? Study design:
The study was double-blind, randomized and with cross-over. This is reasonably rigorous. The authors settled on a sample size of 37 subjects as to attain 80% statistical power, which is “the probability that the test will give the right result when there is a real effect […] and [it] depends on the sample size, and on the size of the effect we hope to detect”. As far as nutritional studies go, this is good. Yet, in the words of David Colquhoun, “The minimum false discovery rate for p=0.05 is seen to be 0.289 [using Berger’s approach]. In other words, if you claim you have discovered something when you observe a p∼0.05, you will make a fool of yourself in about 30% of cases”. Let that settle in for a moment…Statistics aside, the study proceeded as follows.
- 2 week run-in period: all subjects went on a gluten-free (GF) low FODMAP diet.
- Randomization to 3 different diets for 7-days: High-gluten (16g/day), Low-gluten (2g/day) or a whey-control diet (16g/day).
- 2 week wash-out period.
- 3-day rechallenge on 1 of 3 different diets: High-gluten (16g/day), whey (16g/day) or a control diet (0g/day of whey or gluten).
Why diet for this length of time? Does it matter? Rationale for trial lengths:
The authors speak of the 7-day trial and the 3-day rechallenge as 2 separate trials. They give 2 reasons explaining why the rechallenge lasts 3 days. First of all, the original plan was for the participants to stay on each diet for 6 weeks (not 7 days) because “symptoms were uniformly induced within the first week of the original study”. Secondly, the 3 putative gluten responders in the 7-day trial had apparent symptoms within 3 days, thus negating the need for a longer rechallenge period. When discussing possible mechanisms surrounding negative and reproducible effects of gluten, this last point may act as a potential confounder when considering longer-term autoimmune reactions involving molecular mimicry with the thyroid gland or with the nervous system (e.g. cerebellar ataxia).
How do we tell if things got better or worse? Endpoints:
Their primary endpoint was “the change in overall symptom score” on the Visual Analog Scale (VAS) from the 2 week run-in period on GF low FODMAP diet to the treatment-period on 1 of 3 diets. Their secondary endpoints were that slice of participants with a change in overall and individual VAS symptom scores of >20mm as well as markers of protein metabolism byproducts, magnitude of gluten-specific T-cell receptors response, fatigue, activity levels and specifically reproducible GI symptom between the 7-day trial and the 3-day trial.
The easiest person to fool is yourself, so how did they mitigate that? Controls:
They tried to minimize the influence of added food chemicals, they (apparently) successfully reproduced the texture of gluten in gluten-free products and the whey-isolate product was lactose & FODMAP free.
How can we make sure the participants did what they were told to? Adherence:
How was the participants adherence monitored? Daily symptom cards were filled out, significant (>20mm) changes in VAS scores were recorded, notes were taken on fatigue using a daily fatigue scale (D-FIS) and an accelerometer checked activity levels. Furthermore, IgA and IgG specific T-cell responses to gliadin and deaminated gliadin were assayed along with IgE wheat antibodies. Lastly, poop was collected between days 5-7 so to monitor ammonia, β-defensin and calprotectin levels.
So what actually happened? Will gluten shoot your dicks off? Results:
A general trend emerged where overall VAS symptoms improved from baseline to week 2 of the low FODMAP GF run-in period. Only 8 participants (a mere 22% of the total IBS cohort) saw significant improvements of >20mm in abdominal symptoms. The 63% reduction in FODMAPs – from 19g at baseline to 12g during the run-in – may have been insufficient to uncover subtler effects.
Despite this general improvement, symptoms generally worsened on all 7-day trial diets compared to baseline assessments – irrespective of the quantity or absence of whey, gluten or FODMAPs. Considering that FODAMPs were lowered by 86.4-80.5% compared to baseline (down to 2.6-3.7g) across all 3 diets for 7 days, one could reasonably expects commensurate or bigger improvements showing up. Since this did not happen, could the nocebo effect be to blame? The authors suggest it might. This would be because diet content was not associated with degrees of symptomatic responses but diet order was – in both the 7-day trial (A) and 3-day rechallenge (B).
The possibility is reinforced when considering how, in both trials, 1st interventions had mean VAS score changes of 15.5mm whilst 2nd and 3rd ones only changed by 5.3mm and 4.0mm, respectively. In other words, transitioning from the run-in period to the diet-week has an effect that is independent of the kind of diet one transitions to. The fact that only 3 subjects showed gluten specific effects and 7 showed whey-specific ones also argues against an independent diet-content effect. Furthermore, fatigue D-FIS scores presented similarly, showing no noteworthy changes across the 7-Day diets but again, with worse fatigue symptoms when transitioning from the run-in period, irrespective of the diet transitioned to. The authors astutely raise the possibility of “more focused attention to anxiety and depression rather than fatigue might provide additional clues to why patients who follow a GFD feel better”, implying that the D-FIS scale is in fact less appropriate than measures of anxiety and depression. This is strongly supported by the enormity of clinical and anecdotal feedback.
Returning, again, to the order effect and baseline to run-in VAS improvements, these kind of results point to a relatively common sort of response, where gluten may be ‘necessary but not sufficient’ for inducing clinically observable negative effects that have also been reproduced elsewhere, by the very same authors and in other studies.
With the exception of 1 participant with a 3-fold Celiac-like T-cell specific response, that of all other participants was not noteworthy. Neither were the changes in biomarkers obtained from fecal samples. To their credit the authors recognized the discrepancy between their data set and that of others, explaining how “the serological pattern was mostly negative, but there were a lower proportion of cases with positive IgG AGA [emphasis mine] compared with recent data on gluten sensitivity[24]”.
Reference 24 is taken from the results of Umberto Volta et al.’s 2012 paper in the journal of Clinical Gastroenterology, informing us that “[…] IgG AGA were positive in 56.4% of GS [gluten sensitive] patients” out of a cohort of 78.
Interestingly, HLA-D status did not correlate to biomarker changes. I do not have a good enough explanation for this. It is all the more puzzling when one considers that 21 patients on the High-gluten diet, 13 on the Low and another 13 on the whey-control diet were all borderline positive for Whole gliadin IgA values, hovering around 19±3.5U/mol. A negative assay for Celiacs is at <20U/mol. So there is no gradient or dose-response relationship here. Yet, the double-blind placebo-controlled, larger sample study in journal of the American College of Gastroenterology by Carroccio et al. in 2012 demonstrated such a gradient amongst IBS sufferers, wheat sensitive patients and Celiacs. 10%, 40% and 72% respectively tested positive for serum Gliadin IgA. A longer time component, as previously alluded to and exemplified here by Carroccio et al., seems more appropriate for the type of effects being teased out: 4 weeks for the elimination diet, 1 week for between-diet wash-outs and 2 weeks for the single-item reintroduction diets (also with cross-over design).
We’re left with gluten showing no dose-dependent relationship with the severity of symptoms in IBS patients, yet symptoms get noticeably better across the board when FODMAPs are more than halved and gluten withdrawn. What’s more, although the 3-Day Rechallenge gluten & whey-free control diet ‘provoked’ lots of symptoms, all of the gluten containing diets also scored poorly in terms of symptoms.
Ultimately, this study in IBS patients adds to the notion that when gluten is eliminated, symptoms improve – but not just because of gluten. It’s as if it always hangs out in the wrong foods (neighbourhoods) where, when combined with FODMAPs and possibly other components, wreaks havoc. Here FODMAPs seem to have played the role of its context-dependent partner in crime. Knowing the laundry list of other possible offenders in industrialized processed diets, it would be foolish to assume other combinations with gluten won’t produce a vast and continuous (rather than discrete) presentation of symptoms.
All in all, gluten can both stimulate “zonulin, the only known physiologic modulator of intercellular TJs [Tight Junctions] described so far” and cause reproducible immunological reactions (here, here & here). It has both the key to the house and a weapon, its immunoreactive amino acid sequence. What’s more, a genetic model (Celiac disease) gets us lots of supporting information and a ‘most sensitive’ model to help disentangle other symptom presentations. This literal ‘textbook’ explanation of basic immunological mechanisms should help clarify why dismissing gluten as a fad is, actually quite brazen or down right silly.
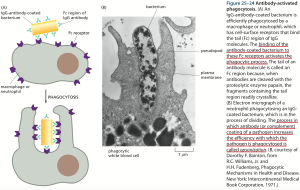
“The clonal selection theory provides a useful conceptual framework for understanding the cellular basis of immunological memory. In an adult animal, the peripheral lymphoid organs contain a mixture of lymphocytes in at least three stages of maturation: naïve cells, effector cells, and memory cells. When naïve cells encounter their antigen for the first time, the antigen stimulates some of them to proliferate and differentiate into effector cells, which then carry out an immune response (effector B cells secrete antibody, while effector T cells either kill infected cells or influence the response of other cells). Some of the antigen-stimulated naïve cells multiply and differentiate into memory cells, which do not themselves carry out immune responses but are more easily and more quickly induced to become effector cells by a later encounter with the same antigen. When they encounter their antigen, memory cells (like naïve cells), give rise to either effector cells or more memory cells (Figure 25–11).
Thus, the primary response generates immunological memory because of clonal expansion, whereby the proliferation of antigen-stimulated naïve cells creates many memory cells, as well as because these memory cells are able to respond more sensitively, rapidly, and effectively to the same antigen than do naïve cells. And, unlike most effector cells, which die within days or weeks, memory cells can persist for the lifetime of the animal, even in the absence of their specific antigen, thereby providing lifelong immunological memory “(p.1546, Chapter 25: The Adaptive Immune System, Molecular Biology of the Cell, 5th Edition, Alberts et al., 2008).
IgG belongs to the cell-surface proteins Ig superfamily. Amongst other tasks, IgG activates the complement system – “complement activation can also greatly increase the immune response to an antigen: the binding of an activated complement component to an antibody–antigen complex, for example, can increase the ability of the antigen to stimulate a B cell response more than a thousand fold” (Figure 25–74, p.1599, Chapter 25: The Adaptive Immune System, Molecular Biology of the Cell, 5th Edition, Alberts et al., 2008).
The Figure ‘Kinetics of Antibody Response’ depicts their 2-humped response pattern and the characteristically long time component of the mechanism within which they operate.
Lastly, consider the Carroccio et al. 2012 study highlights and points from “Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity”
How gluten affects the brain is not clear. It doesn’t seem to need to cross the BBB itself for many of its neuropathological symptoms.
- “In the present study, none of the patients had hypovitaminosis or malabsorption, and more than half of the patients did not even show any duodenal abnormalities. Neuropathologically, there is loss of Purkinje cells and/or degeneration of the dorsal columns (Bhatia et al., 1995; Hadjivassiliou et al., 1998) with facultative lymphocytic infiltration of the cerebellum, dorsal columns and peripheral nerves (Hadjivassiliou et al., 1998)”
Reproducing empirically observable effects with gluten cannot be ignored and is confirmed elsewhere.
- ”Our results clearly showed that a relevant percentage — more than one-fourth — of the patients who underwent DBPC [double-blind placebo-controlled] wheat challenge were really suffering from WS [wheat sensitivity]”
The ‘necessary but not sufficient’ notion stated another way.
- “[…] there was evidence that coexistent triggers, e.g., intestine-damaging drugs or dysbacteriosis, can lead to a more severe intestinal impairment (28 [mouse study – gasp!]). Clearly, wheat antigens may also act in a similar manner”
Whether it is gluten alone or gluten + whatever else is also in gluten-containing products that is not healthy for humans, the gluten-free recommendation stands. This does not include advice to sport a gas mask when walking past bakeries.







My money is on this class of enzyme inhibitors as being the main driver of wheat related problems:
http://www.gastrojournal.org/article/S0016-5085%2813%2900615-X/abstract
http://www.ncbi.nlm.nih.gov/pubmed/23209313
http://www.mdpi.com/2072-6643/5/10/3839
C.
LikeLike
that’s very possibly correct.
i’m particularly interested in gluten because it’s largely through studying it’s effect on the gut that zonulin, the first molecule known to induce gut permeability, was discovered. that makes it a prime candidate.
however, your bet might very well turn out to be correct.
The various inhibitors are definitely very problematic and especially in a FODMAP context. the chicken & the egg question is still very pertinent though and makes this web of causal factors hard to untangle.
LikeLike
Wheat certainly is a hell-brew of avoidable substances. I’m not sure where wheat germ agglutenin fits into this picture either. Interestingly, the ATI’s mentioned in those papers were isolated from Gliadin and re: zonulin, wikipedia states “Gliadin (glycoprotein present in wheat) activates zonulin signaling irrespective of the genetic expression of autoimmunity, leading to increased intestinal permeability”, http://www.ncbi.nlm.nih.gov/pubmed/21248165?dopt=Abstract, so perhaps these two ideas converge?
Maybe it is a little confusing to talk of gluten specifically — my understanding is that gluten is a cross -linked product of glutenin and gliadin due to mechanical and chemical processing. It is possible to isolate gluten from everything else including the ATI’s by making seitan (e.g. http://www.onegreenplanet.org/vegan-food/how-to-make-perfect-seitan/) and then to investigate whether or not that causes health problems. But a detached clinical interest seems to be the safest approach!
Craig.
LikeLike