During the upper-middle Neolithic era, about 1500 years B.C, a first description of diabetes as a “too great emptying of the urine” appeared in Egyptian manuscripts. Since then it has suffered from further flimsy nomenclature, attesting to the medical and scientific challenges posed in characterizing its murky & complex etiology. This is somewhat justified by the fact that diabetes carries a disagreeable trio of origins; genetics play a non-trivial role in its incidence (about 10% of cases), it has an autoimmune presentation (Type I Diabetes) and most importantly, lifestyle factors contribute the largest chunk on a population basis (i.e. it is a disease of civilization for the most part). Science however is about making the complex simpler and in the 1960s Peter Cleave did just that by describing diabetes as ”the saccharine disease because he believed sugar and other refined carbohydrates were responsible”. Although scientifically incomplete, it remains a fair and useful heuristic-like description.
If you are diabetic, you will either be a Type I (autoimmune), a Type II or if you also happen to be pregnant you might fit into the (transient) Gestational Diabetes category. A diagnosis is made on the basis of:
- elevated fasting blood sugars (before at >140mg/dL and currently lower at >125mg/dL)
- ongoing hyper and/or hypoglycaemic episodes as well as elevated 3 month blood sugar averages (HbA1c ≥ 6.5%, calculated as a % of non-enzymatically glycated hemoglobin). Note that insulin measures are not necessarily part of the diagnosis, one important point which we will get back to.
Most diseases are traditionally viewed as discontinuous qualitative entities but diabetes is not so. The term pre-diabetes implies that the diabetic state is in fact continuous and thus quantifiable. To be considered pre-diabetic one only needs to have fasting blood sugars at or above 100mg/dL.
To get a sense of what proportion of a population this can include nowadays, the 2014 age-adjusted World Health Organization (WHO) estimate considers 22.4% of people in Bangladesh to be pre-diabetics. Before the biochemistry of diabetes is explored further, it is helpful to note that most physicians now consider oral glucose tolerance tests (OGTTs) the gold standard for assessing a patient’s location on the diabetic spectrum rather than fasting blood glucose measures. How one handles a 75g liquid bolus of glucose over the following 2 hours holds better predictive power than fasting glucose measures do because this post-prandial mimic better accounts for the interplay of glucose management as a function of insulin dynamics. However this test is also far from perfect. It fails to distinguish between generalized pathological insulin resistance characteristic of diabetes and non-pathological tissue-specific insulin resistance. For example, it can produce false-negatives by failing some individuals on low-carbohydrate or ketogenic diets. This is thought to happen because of non-pathological peripheral insulin resistance which prioritises glucose produced from the liver or coming in from the diet to serve the human brains’ minimal ~40% need for glucose-derived energy; the musculoskeletal system does equally well (if not better) on fatty acids and ketones and can thus become somewhat insulin resistant to manage tissue-specific and whole-body energy homeostasis.
A simple descriptive model of diabetes provides context for grasping its finer intricacies and contentious mechanistic points. When carbohydrates from a meal are absorbed through the intestines they go on to circulate in the blood. They reach various organs like the heart, muscle, liver, brain and non-organ cells like adipocytes. Insulin is (mainly) secreted in response to carbohydrates and performs its fundamental job of getting them (as well as proteins and fats) into cells. Diabetes results when insulin’s action is insufficient for managing the carbohydrate load by getting most of it into various cells and thus out of the blood stream. This results in higher levels of circulating glucose in the blood, namely hyperglycemia. Depending on whether the diabetic is a type I (T1D) or II (T2D), hypoinsulinemia and hyperinsulinemia develop over the long term, respectively.
The resolution of this simplified insulinocentric model can be sharpened in both depth and breadth by integrating 3 more angles. First and most importantly, is to integrate the endocrinological dynamics of insulin with the metabolism of cellular energy management. This should help relate the interplay right down to the flux of energy currency molecules between the cytoplasm and mitochondria as well as those molecules that handle them. Secondly, some thought will be dedicated to insulin’s ‘opposing hormone’ glucagon which will refine the diabetic model, the corollary of which is to render it less insulinocentric. Lastly, the quite mysterious influence of the microbiome upon glucose and whole body energy homeostasis is gaining undeniable scientific prominence due to the alluring correlations it has so far presented. An attempt is made to show how these 3 angles are strongly dependent upon lifestyle factors which includes but is not limited to; nutrition, sleep and movement (or exercise). Each ‘angle’ is differentially understood and does not contribute to the etiology and phenotype of T2D equally. Quantifying each ones apportioning to it is the ball of yarn that has yet to be untangled.
For glucose to get into myocytes, insulin must be present within the blood in sufficient concentrations as to bind its tyrosine kinase insulin receptors (isoform IR-B mainly) that are found within plasma membranes and stick out of the cell surface. Insulin acts as a ligand here. The insulin-binding domain of the receptor is formed by 2 α chains which are the extruding part of the receptor. Its intracellular domain is composed of 2 transmembrane β chains subunits which have carboxyl termini protruding into the cytosol. When the α chains are activated, autophosphorylation of both of the β chain subunits takes place. 2 αβ monomers form upon α chain activation where 3 tyrosine residues on each β chain become phosphorylated by the other β chain. This has for effect of displacing the activation loop 30Å away from the substrate-binding site it was previously occupying, now enabling target proteins to access the site. In turn, insulin-receptor substrate 1 (IRS-1) gets phosphorylated by having a phosphoryl group from adenosine triphosphate (ATP) transferred to the hydroxyl group on its Tyrosine residues (since the initial stimulus comes from insulin in this scenario). Phosphorylated IRS-1 is a nucleation site – somewhat acting akin to a sorting junction – from which protein complexes go on to carry out messages down into the cytosol and nucleus, affecting GLUT4 translocation and gene expression, respectively. GLUT4 translocation happens through a variety of interweaving and still incompletely understood pathways. GLUT4 translocation to the plasma membrane can happen in a phosphatidylinositide 3-kinase (PI3K)-dependent or independent manner. PI3K is a signal transducing enzyme activated by G-protein coupled receptors or tyrosine kinase receptors. Muscle contractions can stimulate GLUT4 translocation in a PI3K-independent manner affecting whole body glucose homeostasis, making it relevant to diabetes and general health. Here however, we consider and continue on from phosphorylated IRS-1 in an insulin stimulated GLUT4 translocation scenario engaging the requisite PI3K. The diagram below depicts phosphorylated IRS-1 forming a complex with PI3K.
PI3K is composed of 2 subunits bound together; regulatory and stabilizing subunit P85 and catalytic subunit p110. p85’s inhibitory activity on p110 is disabled via phosphorylation of its tyrosine residue located on the amino-terminal of its SH2 domain. This activates p110’s catalytic activity and translocates PI3K to the cell membrane where its substrates reside (see ‘Fig.2 The key molecular signals that are turned on and off by insulin regulating GLUT4 traffic’). PI3K phosphorylates it residue phosphatidylinositol-4,5-bisphosphate (PI[4,5]P2), producing phosphatidylinositol-3,4,5-trisphosphate (PI[3,4,5]P3). A key point worth stressing is that the amplitude and time component of the stimuli acting to produce PI(3,4,5)P3 can determine the extent to which and whether or not GLUT4 translocates to the plasma membrane. This point may be an important determinant in the development of insulin resistance since the pulsatility of macronutrient intake (frequency and amplitude) provides important energy availability signals to the cellular milieu in addition to satiety and satiation cues to neuronal circuitry.
Returning to the pathway at hand, the phosphorylated PI3K substrate PI(3,4,5)P3 in turn “recruits Akt1/2 [Protein kinase B] to the PM [plasma membrane] and possibly to endomembranes, where the enzyme [Akt1/2] is phosphorylated on the Thr308/309 residue of its activation loop by 3-phosphoinositide-dependent kinase (PDK1)”. It appears Akt2 rather than Akt1 is predominantly involved in this process. Isoforms λ and ζ of atypical Protein Kinase C (aPKC) are only stimulated by insulin and participate in GLUT4 translocation by being recruited to the PDK1/Akt complex, effectively forming a phosphorylated trio. It should be noted that the downstream effectors of PI3K (Akt and aPKC) control GLUT4 translocation both from within intracellular compartments and at the plasma membrane. This is evidenced by the arrow leaving PKC-λ/ζ leading to GLUT4 translocation and by it being complexed to PDKI/Akt visible in ‘Fig.2 The key molecular signals that are turned on and off by insulin regulating GLUT4 traffic’. The IRS-1/PI3K and Akt/aPKC-λ/ζ complexes form by co-purifying (meaning they attract each other) to cellular subfractions enriched in GLUT4 in order to enact its translocation to the plasma membrane. It is still uncertain whether aPKC is necessary for GLUT4 translocation or if Akt alone is sufficient for insulin-stimulated glucose uptake. In any case, a 160kDa protein substrate target of Akt (AS160) acts as a figurative ‘brake’ on insulin-stimulated GLUT4 translocation when its GTPase-activity protein (GAP) domain is in its active form and maintaining its yet unidentified target Rab protein in GDP form. Rab proteins are a branch of the Ras GTPase superfamily of proteins steering intracellular membrane traffic. When 5 of 6 of AS160’s target sites for Akt are phosphorylated, its GAP domain is now inactive. This means its target Rab is phosphorylated and thus changed to GTP form, now rendering it capable of partially inducing GLUT4 translocation from both ERC/TGN (endosomal recycling compartments/trans Golgi network) and SC (specialized compartments) compartments. In vivo data from mice suggest Rab10 is the likely Rab target of AS160. GLUT4 buds off in endomembranes (erroneously termed vesicles), making its way to the plasma membrane where it can catch glucose molecules floating around the cell surface. GLUT4 then mediates the entry of glucose molecules within the cell’s cytoplasm. When insulin levels decrease sufficiently, GLUT4 transporters are removed from the plasma membrane by endocytosis. In so doing, an endomembrane is formed so that the GLUT4 transporter can make its way back inside the cell and fuse with other ones, forming a larger endosome which effectively completes its cycle.
The importance of insulin signaling amplitude and frequency (previously referred to as pulsatility) is reiterated, in so many words, by authors Grant and Donaldson, saying:
“The balance between endocytic uptake and recycling controls the composition of the plasma membrane and contributes to diverse cellular processes including nutrient uptake, cell adhesion and junction formation, cell migration, cytokinesis, cell polarity and signal transduction. Since it is estimated that cells internalize their cell surface equivalent one to five times per hour 1 [my emphasis], endocytic recycling pathways must be robust and coordinately regulated.”
At this point it is clear that what and when one eats is front and center in the development and reversal of Type II Diabetes (T2D). The etiology of Type I Diabetes (T1D) however, is immune mediated, so food plays more of a direct role in its management rather than development, as attested to by T1D doctors and athletes. Circadian rhythms compete with food in this hierarchy of factors since they are biological metronomes orchestrating membrane traffic and receptor sensitivity, amongst other tasks. Simply put, food and sleep (1,2) are at the root of prevention, management and reversal of the metabolic syndrome and diabetic phenotype. At last, the discovery of AS160 in the last 10 years provides an exciting new candidate that may possibly lend mechanistic power to another factor in diabetes, namely movement. It presents as a ‘missing link’ or common pathway for both insulin and exercise-stimulated GLUT4 translocation in myocytes capable of affecting whole body glucose homeostasis. This would go some way in explaining the effectiveness of lifestyle interventions over pharmacological ones for diabetes management because the same pathways are exploited from multiple stimuli. Nevertheless, it is important to realize that despite exercise being a tool for improving insulin resistance, it has never been shown that one can ‘out-exercise’ a poor diet or sleep debt.
Getting back to the sequence of molecular events described above, these are specific but not necessarily exclusive to muscle cells. It is but a snapshot of an incredibly more complex overarching process. The point is driven home by Table 11-4 which lists tissue-specific glucose transporters.
The kind of insulin receptors and isoforms directly affect glucose metabolism and thus diabetes, in addition to also being implicated in certain cancer phenotypes. The genetic and non-genetic (herein referred to as epigenetic or exposomic) influences on insulin are presently innumerable. Insulin resistance characterizes pathological insulin dynamics and may well be the single biggest lever determining human health and disease. This notion is reinforced in Table 15-3, where insulin’s wide influence on genes and pathways is listed.
Cynthia Kenyon’s work with C. elegans adds support to the importance of insulin and insulin response elements by identifying the influence of orthologous genes daf-2 and daf-16 (IGF-1 and FOXO respectively in humans) which strongly impacting ageing and the development of diabetes, respectively. One pathway via which ageing and diseases manifestation are thought to be influenced is simplified in her mouse-human diagram.
It delineates a cascade of interactions between growth hormone (GH) and its receptor (GHr), insulin-like growth factor 1 (IGF-1), insulin receptor substrate protein (IRS), Akt and the forkhead family of transcription factors (FOXO). Together they play a role in communicating messages of apoptosis or new growth and repair at the cellular level which not only has obvious implications in cancers but also in diseases of accelerated ageing like diabetes (which will be explored further). Our understanding of how this is mediated is still unclear though. For example, although FOXO is associated with centenarians, the strongest FOXO single nucleotide polymorphism (SNP) association is weak statistically speaking, with an odds ratio (OR) of 1.42 (CI 1.18-1.70), to which the authors caution, saying “…the large confidence intervals for the ORs in both studies imply some uncertainty”. There are stronger longevity measures assessing the impact of diabetes such as the 2010 British report, which estimates that T1D’s live 20 years less and T2D’s 10 years less on average. As evidenced in Table 2, the role of growth hormone and related components of the insulin pathway offer contradictory evidence as it pertains to longevity.
It does however give every indication that the insulin pathway and its components are levers for both and longevity and health.It does however give every indication that the insulin pathway and its components are levers for both and longevity and health. It has yet to be understood how particular actioning of these levers goes on to manifest a diabetic phenotype.
Amino acids and pyruvate, the latter resulting from cytosolic glycolysis (catabolism of glucose), are transported from the cytosol into mitochondria where they feed into the Citric Acid Cycle (TCA cycle). It takes place on the outer mitochondrial membrane where two-thirds of carbon compounds are oxidized in human eukaryotic cells. Fatty acids and ketone bodies circulating in the blood stream are also imported into the mitochondria where they undergo oxidation on the inner mitochondrial membrane along with products from the TCA cycle. These 3 reactions effectively feed into each other, starting with cytosolic glycolysis, leading into the TCA cycle and ending with oxidative phosphorylation. Most of the daily energy derived in humans is derived from fatty acid oxidation. The TCA cycle produces 3 kinds activated carrier molecules NAD+-NADH (oxidized and reduced nicotinamide adenine dinucleotide), FADH2 (reduced flavin adenine dinucleotide) and ribonucleotide GTP (guanosine triphosphate) which all participate in generating ATP that cells and their tissues need at all times for proper function. NADH is also used directly by the cell for energy. Glycolysis and the TCA cycle produce intermediates used in cell building (biosynthesis) in addition to providing raw metabolic energy materials. Of particular interest in diabetes is the balance of ATP and activated carrier molecule ‘pools’ cells need to continuously maintain, as well as the array of molecules handling these. How diabetic neuropathies manifest can serve to characterize their roles.
Table 2 by Chowdhury et al. lists some of the aberrant mitochondrial physiology found in certain diabetic tissue/culture models.
In many cases, respiration is compromised from a variety of points. This feature is eerily similar to respiratory deficiencies characteristic of cancer cells. Thiazolidenodione diabetic drugs aim to increase deficient peroxisome proliferator-activated receptor [PPAR] γ co-activator 1α (PGC1-α) activity since PGC1-α stimulates mitochondrial biogenesis. PGC1-α is highly expressed in energy intensive tissues such as skeletal muscle, brain, heart, liver and brown adipose in addition to during energy demanding activities like being cold, exercising and fasting. PGC1-α is a potential molecular link for explaining why, for example, fasting was such a popular intervention for non-insulin dependent diabetics in the pre-insulin era as well as its present day re-implementation.
Elliott P. Joslin explains the clinical significance of fasting and macronutrient restrictions:
“Such individuals [diabetics] should be taught to regulate the quantity of food eaten by the body weight, and never to indulge in unusual quantities of carbohydrate. […] That temporary periods of under-nutrition are helpful in the treatment of diabetes will probably be acknowledged by all after these two years of experience with fasting. In no other way can one so readily keep the urine free from sugar and this is the foundation of all diabetic treatment.”
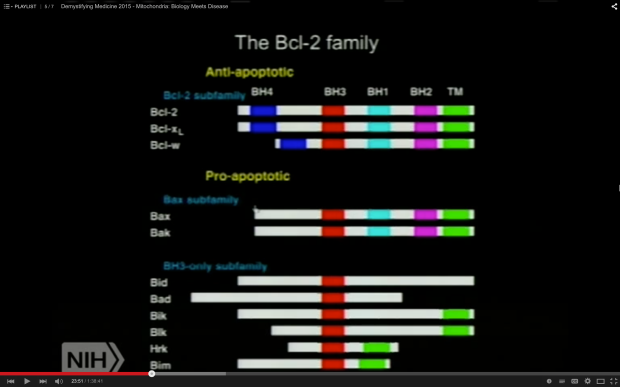
Chowdhury et al. make the point that “apoptosis-dependent loss of sensory sympathetic neuron perikarya has not [emphasis mine] been found in diabetic humans or animals” but that “mutation or loss of proteins associated with mitochondrial function can lead to neuropathy that mimics that seen in diabetes, for example, loss of function of mitofusin-2 or bcl-w results in a length-dependent sensory fiber degeneration.” As shown in the screenshot of an NIH video presentation, Bcl-w has an anti-apoptotic role, regulating mitochondrial turnover.
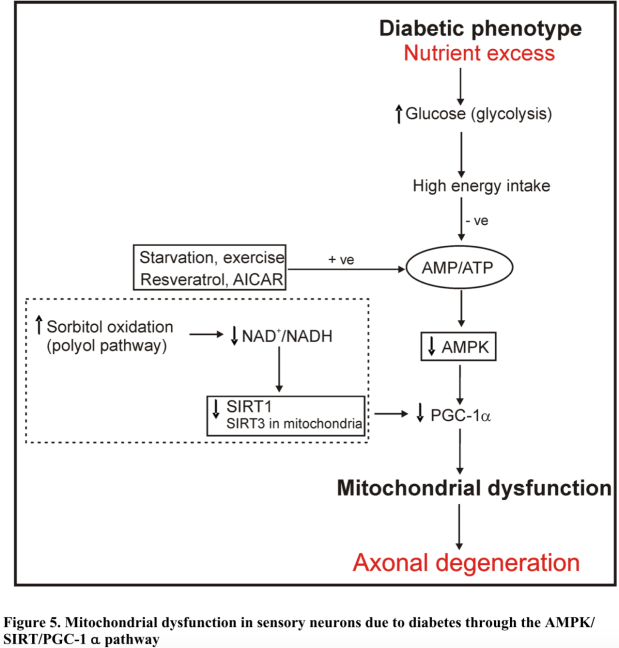
Multiple proteins involved in the latter present as strong candidates for pin-pointing molecular disturbances in people with diabetes and metabolic syndromes. It appears that a causal link for mitochondrial dysfunction runs from 5′ adenosine monophosphate-activated protein kinase (AMPK), NAD-dependent deacetylase sirtuins (SIRT1 and 3) and PGC1-α. AMPKinase senses the ATP/ADP ratio and in a state of excess glycolysis that ratio is increased such that enough AMP turns into ADP via substrate-level phosphorylation, causing PGC1-α to downregulate. PGC1-α can no longer prompt adequate mitochondrial biogenesis (as seen in Fig.5).
Furthermore, cytoplasmic enzyme SIRT1 can sense a hyperglycemia-induced depressed NAD+/NADH ratio. This is often secondary to insulin failing to maintain intracellular glucose homeostasis. This reduces SIRT1’s activity, thereby lowering that of PGC1-α’s, which decreases the rate of mitochondrial biogenesis. The corollary of which is that faulty mitochondria are not adequately repaired or repurposed by autophagosomes. SIRT1 has far reaching impacts on FOXO, PPARγ, PGC1-α and tumor protein 53 (p53).
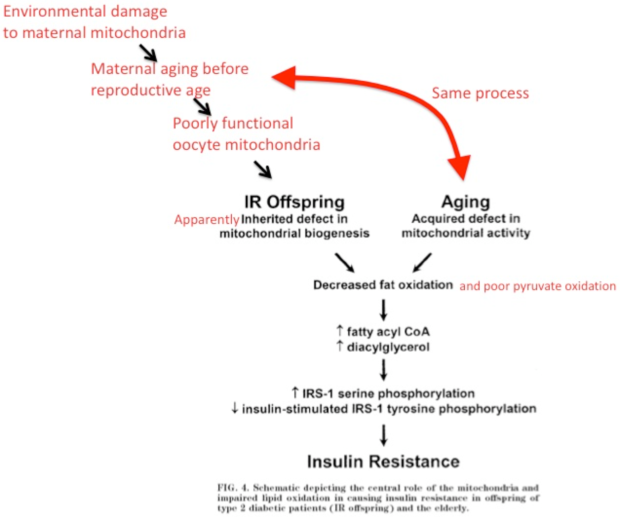
Fig.4 is edited by Royal Veterinary College trained veterinarian Peter Dobromylskyj and it schematizes the development of insulin resistance in T2D patients by linking faulty mitochondrial action and impaired lipid oxidation.
For all intents and purposes, the type and manner of fuel use resulting from these molecular cascades resembles the Crabtree effect. In effect, increased glycolysis produces abnormally high levels of ATP through substrate-level phosphorylation which essentially ‘tells’ the cell that TCA cycle oxidative phosphorylation run through the electron transport chain (ETC) is no longer as necessary. Hyperglycemia begets further hyperglycemia. Oxygen consumption is thus reduced. Interestingly, less superoxide (O2–) was shown to be associated with T2D in rats. In contrast, more ROSs overall are usually implicated as a hallmark of mitochondrial dysfunction. Which ROSs and the reason for them not being handled by anti-oxidants may provide important clues as to the precise etiological route in one diabetic versus another. This logic may apply to the development of other metabolic syndromes.
The complex picture interweaving molecular biology and endocrinological mechanisms can easily overshadow the necessity of establishing a sufficiently simple and solid clinical picture for practitioners to guide them in patient care. Thankfully but also tragically, Dr.Joseph Kraft appears to have succeeded in the latter to a great extent, all the while having his findings mostly ignored by the wider medical profession. From 1972 to 1989, Dr.Kraft gathered empirical clinical data on his patients (aged 3 to 91 years old) by performing a glucose tolerance test in conjunction with an insulin assay. Done together, the earliest signs of diabetes and insulin resistance can be identified. This level of diligence and rigor is rare, even by modern standards. During this time period he performed an astonishing 14,383 tests. 95% (2,635) of his patients out of the 2,775 identified as glucose intolerant had T2D, 1.98% (55) had T1D and 2.99% (83) did not have diabetes. 74% (7,102) of his patients out of the 9,598 identified as normally glucose tolerant had T2D hyperinsulinemia, 22% (2,112) did not have diabetes and 4% (384) had pre-hyperglycemic T1D hypoinsulinemia. Dr.Kraft’s several thousand autopsies suggested to him that diabetic pathology is vascular. This observation was also espoused earlier on by Dr.Stout’s from the 1970s onwards due to his experiments strongly implicating the need of insulin action upon the endothelium in order for it to become ‘injured’.
Table 1 lists selective glucagon receptor antagonists that are in phase 1 and 2 clinical trials.
In large part, the main resurgence of interest in interventions for diabetes targeting glucagon stems from the ugly fact that T1D and far-progressed insulin-dependent T2D is poorly managed by insulin injections. Granted, low carbohydrate-high fat and ketogenic diets greatly reduce the need for insulin injections and broaden this interventions’ margin of error. Nevertheless, this balancing act remains difficult and perilous. The bihormonal (insulin and glucagon) rationale for the management of diabetes is partly based on 6 observations recounted by Robert Unger and Alan Cherrington:
- insulin deficiency is associated with catabolic events that are partially mediated by glucagon, such as hepatic glucose and ketone production
- whichever form of poorly controlled diabetes, it is always characterized by hyperglucagonemia
- leptin and somatostatin are glucagon suppressors and they manage to suppress the catabolic manifestations occurring during insulin deficiency
- glucagon suppressors leptin and somatostatin suppress all catabolic manifestations of diabetes during total insulin deficiency
- total destruction of β-cells in -/- glucagon receptor mice does not cause diabetes (a truly inconvenient and reproducible result which the insulinocentric model of diabetes cannot explain)
- when the pancreas of rats are perfused with anti-insulin serum this engenders severe hyperglucagonemia
In addition to these observations, a known but poorly addressed issue in insulin-dependent diabetes protocols is plasma insulin ‘concentration disparity’. Subcutaneous insulin injections go into adipose and skeletal tissues as well as the liver and pancreatic α and β islet cells. These all require different concentrations of the hormone. This blanket approach “results either in underinsulinization of α cells or in over-insulinization of peripheral tissues [as depicted in panels A, B and C]”.
These lines of evidence supporting glucagon’s bihormonal importance in the management of diabetes are partially reflected in a 1975 New England Journal of Medicine study of 7 human patients with juvenile-type diabetes (T1D). It would most likely be impossible to replicate nowadays. It showed that these patients did not develop diabetic ketoacidosis for 18 hours after acute withdrawal off of insulin as long as glucagon was suppressed from pancreatic α cells using somatostatin-14. The latter form of somatostatin has higher affinity with pancreatic α cell receptors while somatostatin-28, which is released upon intake of dietary fat, has higher affinity with pancreatic β cell receptors. When β cells are non-functional, as was essentially the case in this study, a high-fat diet will partially suppress glucagon, albeit in a relatively weak manner due to somatostatin-28 release acting on α cells.
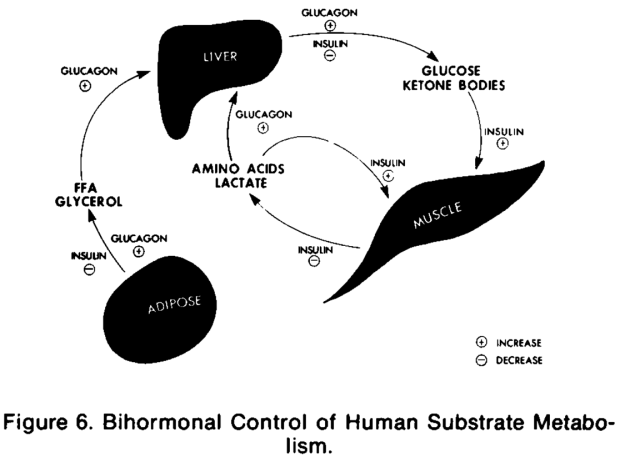
The authors schematize the mechanism in Figure 6 whereby insulin’s action is secondary in importance to that of glucagon’s and they describe the implication of their result, explaining how “insulin per se does not lead to diabetic ketoacidosis in man and that glucagon, by means of if its gluconeogenic, ketogenic, and lipolytic actions, is a prerequisite to development of this condition.”
Beyond the hormones insulin and glucagon, the human microbiome presents itself as potentially novel player of note impinging upon whole body glucose and energy homeostasis. The last 10 years have heralded the emergence and importance of the human microbiome which now also adds an astounding layer of complexity to diabetes. A 2012 study by Vrieze et al. showed that within 6 weeks of a fecal microbiota transplantation (FMT) from lean donors to obese recipients with metabolic syndrome, the obese recipients displayed improved insulin sensitivity as measured by “a median rate of glucose disappearance […] from 26.2 to 45.3µmol/kg/min; P < 0.05”. The mechanisms mediating this are uncertain. The statistically significant (P < 0.05) changes in butyrate-producing bacterial species in the proximal and distal intestines suggest to the authors “that butyrate produced by certain bacteria prevents translocation of endotoxic compounds derived from the gut microbiota which has been shown to drive insulin resistance”.
The diagram of Udayappan et al. depicts 2 ways in which “altered [i.e. less Short Chain Fatty Acid-producing] gut microbiota composition may affect the host metabolism via impaired intestinal barrier function resulting in low-grade endotoxaemia”.
It is important to note that the statistically significant changes in bacterial populations in the Vrieze et al.-FMT study should not be assumed to biologically significant long-term – not until further replication and supporting data is seen. Nevertheless, the changes in glucose metabolism seen after FMT interventions are striking and so far do not suggest they would bring with them the brutal and numerous side affects of popular glucose lowering agents.
There is an irony whereby conventional medicine both espouses insulinocentrism in many respects yet concomitantly fails to appreciate its veritable actions and wider relevance. The insulinocentric models of diabetes does need broadening and refining. The bihormonal approach to diabetes sharpens the classic endocrinological picture. Renewed appreciation for the powerful effects of food, sleep and exercise (otherwise known as lifestyle factors) capable of altering the diabetic phenotype revives effective late 19th and early 20th century interventions. The microbiome is posed to help fill-in knowledge gaps about diabetes and metabolic syndromes by characterizing the nature of the relationship between our organism and microbiome. It can be concluded that the preponderance of evidence points to insulin as being the biggest single lever in the etiology and reversal of diabetes. However, insulin can be acted upon by many inputs, reflecting its central endocrinological and metabolic role in regulating whole-body energy homeostasis. There is more than mere hope for culling the impact of diabetes worldwide.












This is a great reference Raphi. On the last point you might find this new study of vinegar and glucose tolerance has insights into the butyrate effect, acetate and butyrate having similar inputs.
https://www.dropbox.com/s/1pdt3xw0c1u70xx/Vinegar.pdf?dl=0
This is quite a good introduction to how leptin and other appetite hormones interact with the insulin-glucagon axis and microbiota
http://www.freetree.co.com/carbohydrates-and-leptin-resistance-via-intestinal-bacteria/
If leptin also inhibits glucagon, what is its relation to dietary fat?
most studies regarding the relationship between high-fat diet and concentration of leptin were found that there is a positive association between intake of higher fats and leptin level.
One cross-sectional study conducted among individuals with type 1 diabetes had shown that men consumed more SFA had more concentration of leptin.
High-fat diet substantially enhanced plasma level of leptin in rats.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4251481/
But compared to somatostatin 28, not so consistent a relationship with fat. Still, every little bit helps suppress DKA.
LikeLiked by 2 people
Thanks George!
I saw you posting that & will have to get around to it seeing as I’m a vinegar fiend. Thanks for the leptin links – i’m not as familiar with it as with other hormones so these are appreciated.
There’s so much more I wanted to write about but was constrained by word limits. I’ll have to make more of an effort to include leptin in the future as ItsTheWoo makes convincing points regarding its relevance to metabolic syndromes.
LikeLike
Um…wow. I’m going to have to read this 2 or 3 more times to fully understand it. (If I’m even *capable* of understanding it!) This must’ve taken you a long time to put together. Thanks for your work!
LikeLiked by 1 person
Thanks Amy!
Yes, quite a while 🙂 …I learn better by writing than plain reading – that means 10 ‘units’ of reading for every writing unit. As you can imagine, it leads one down many rabbit holes and it becomes harder to maintain a simpler, bigger picture view. Even here, I only explored 1 pathway for 1 kind of glucose transporter in 1 kind of cell! That’s also why I think one needs heuristics, however limited they are in accuracy. I tend to learn & base my arguments on 2 very different levels of ‘resolution’: the very minute & specific (e.g. redox mechanics between electron carrier molecules) and the very big & general (e.g. evolutionary adaptation arguments married to empirical data like ‘they eat this & feels better/worse’). The trick is linking them, of course 🙂
LikeLike
Wow. Thanks for taking the time to put this together! I’ll print it out and will read it in bed 🙂 .
LikeLiked by 1 person